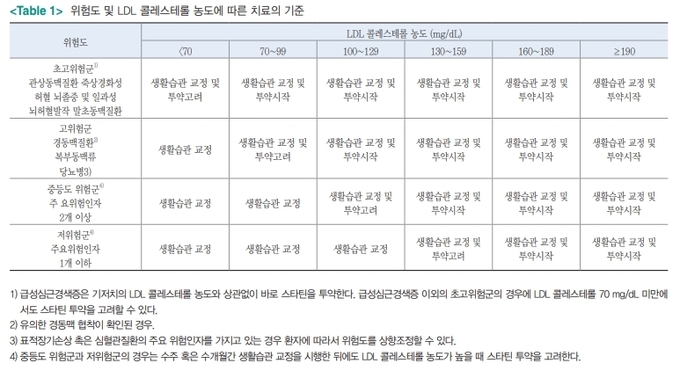
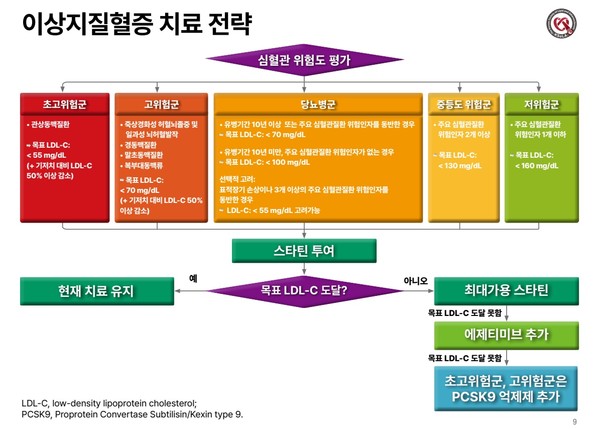
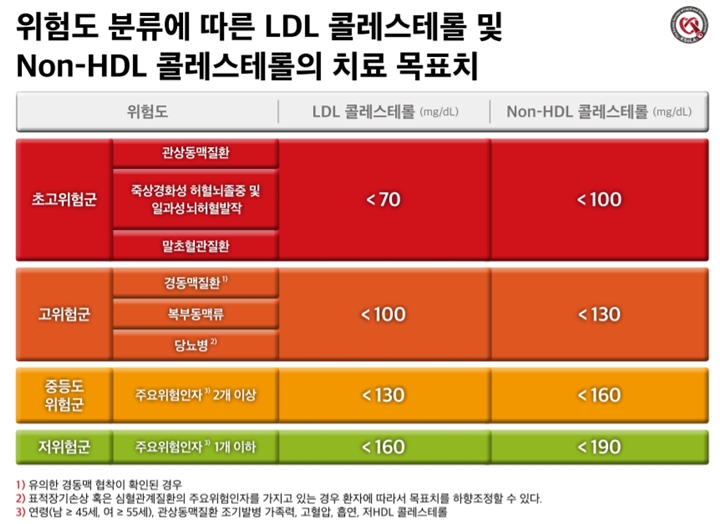
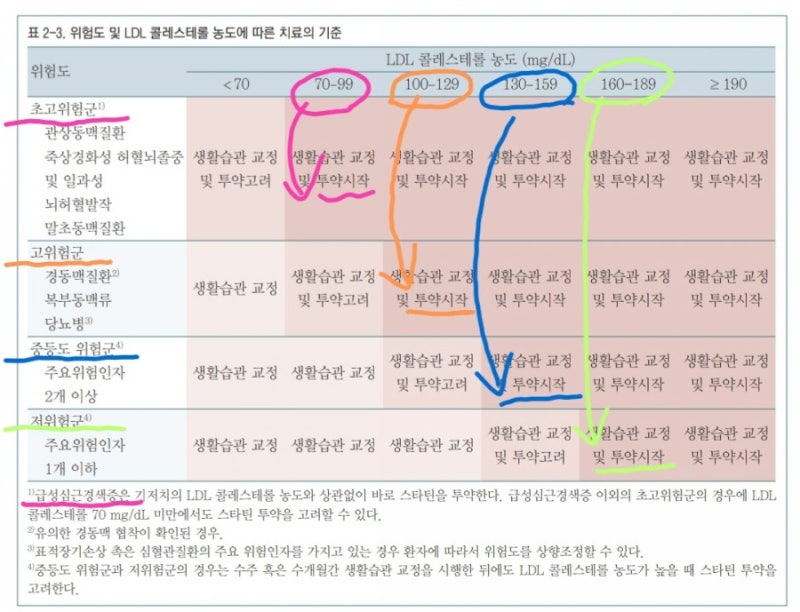
mortality risk factor (relative risk factor)
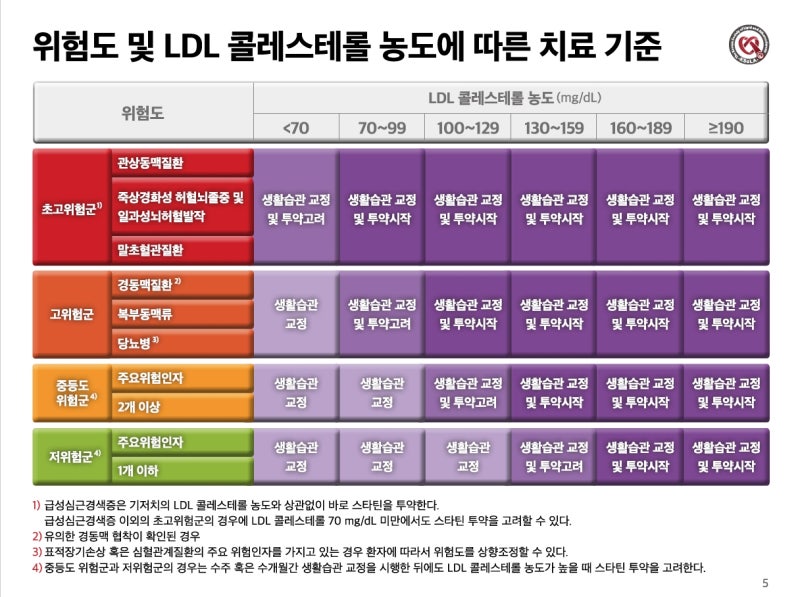
Age 18-39 years 1 (Criteria) 40-49 years old 2.250-64 years old 4.365-74 years old 6.775-84 years old 8.5>85 years old 10.6 obesity 1.3 underlying diseases Diabetes, complications Chronic kidney disease Chronic lung disease Cardiovascular disease 1.31.21.1
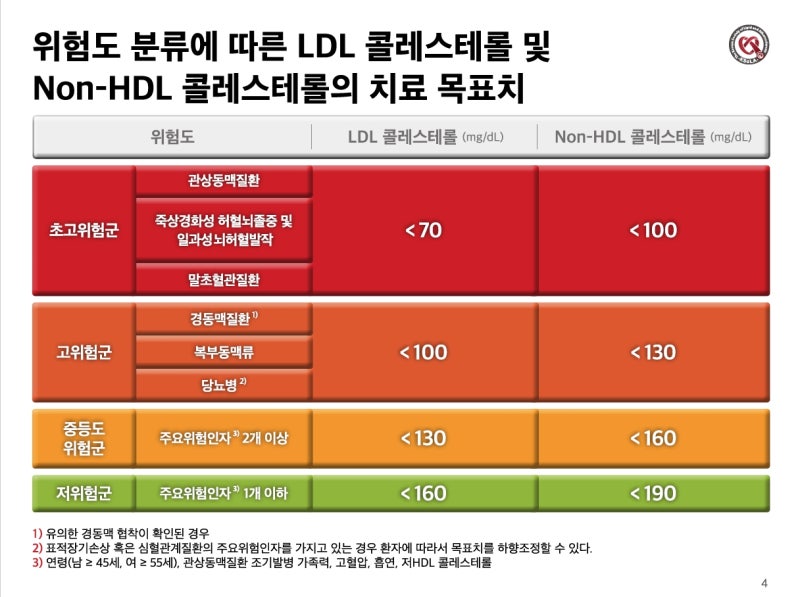
severity classification

Minor symptoms (upper respiratory tract infection) No pneumonia detected, no oxygen treatment required Moderate pneumonia (lower respiratory tract infection) but severe pneumonia with no oxygen administration, oxygen saturation less than 94%, PaO2/Fio2 <300 mmHg respiration rate more than 30 times, pulmonary invasion more than 50%, septic shock, multiple organ failure

Minor symptoms (upper respiratory tract infection) No pneumonia detected, no oxygen treatment required Moderate pneumonia (lower respiratory tract infection) but severe pneumonia with no oxygen administration, oxygen saturation less than 94%, PaO2/Fio2 <300 mmHg respiration rate more than 30 times, pulmonary invasion more than 50%, septic shock, multiple organ failure
classified severity classification criteria ANTIVIRAL AGENT IMMUNOTHERAPEUTIC AGENT Fever, RESPIRATORY SYMPTOMAIN DIFFICULTY PULMON INFILTRATION OXYGEN SATURATION DEGREE OF 94% OR LOWER THERMOBILIZATION REMDIBRIODEXAMETHASON BARICITYNIP ANTI-THROMBRES 0 × × × × × × ××× Secondary 000 Severe patient 00000 △ × 0X0X0
classified severity classification criteria ANTIVIRAL AGENT IMMUNOTHERAPEUTIC AGENT Fever, RESPIRATORY SYMPTOMAIN DIFFICULTY PULMON INFILTRATION OXYGEN SATURATION DEGREE OF 94% OR LOWER THERMOBILIZATION REMDIBRIODEXAMETHASON BARICITYNIP ANTI-THROMBRES 0 × × × × × × ××× Secondary 000 Severe patient 00000 △ × 0X0X0
1. 10 days for mild diseases, 1 week for self-isolation confirmed patients, 2 PCR negative tests for uninfected patients, and efforts to prevent infection and spread: Medical facility staff wear personal protective equipment and social distancing

Tylenol ibuprofen Capacity 10-15 mg/kg Adult 325-1000 mg, Maximum 4 g/day 5-10 mg/kg 12 years of age or older 200-400 mg, Maximum 2.4 g/day daily routine 4-6 hours, 6-8 hours as needed
4)Throat pain Tantum/betazine/crohexadine gargle 5) itching, rash antihistamine (phenylamine, ziltech) oral steroid interferes with antibody formation during initial viral discharge 2. Follow-up and conservative treatment for diseases above moderate diseaseinitial infection (first week) Lung infection host immunity/inflammation (after 1 week) time of viral growth timing of host immune response ANTI-CORONAVIRUS THERAPEUTIC AGENT IMMUNOREACTION REGULATOR (STEROID)3. Therapeutic agents1.Combination of virus target (spike protein) antibody treatments 2.cellular target carostat 3.viral proliferation antiviral agents (remdesivir, paxlovide, dexamethasone) 4.drug resident mutations antibody treatments1.Combination of virus target (spike protein) antibody treatments 2.cellular target carostat 3.viral proliferation antiviral agents (remdesivir, paxlovide, dexamethasone) 4.drug resident mutations antibody treatmentsANTIUMANIC AGENT FOR DRIVERIAL AGENT FOR DRIVERIAL INDIMAL ANIMALYZERIAL INDUCTION OF DRIVERIAL INDIMALYDROXY,low dose LMWH(therapeutic doseに増量)enoxaparin 40mg/30qd CrCldalteparin 5000u qdnadroparin 3800/5700u qdkg1) A patient with a patient who is not breathing difficulty, a secondary body of 20 mgqd for patients who have not been patient with a fever and a severe progressive period of 20th, and a dielectric constant treatment period ofFor patients who are administered on the patient’s symptoms of patients, and the secondary disease, and the secondary disease (for example, 60) of the secondary disease and a secondary disease (for example, or more than 40) of4)Remdesivir (Antiviral, Bechuri)Approval for emergency use of existing indications (21.1.20) Adults and children aged 12 or older over 40kg fall under any of the following three conditions (22.1.7 days expanded) 1. Hypoxia (94% oxygen saturation) 2. Pneumonia (thoracic radiation or CT) 3. 200mg from 2nd day* Monitoring: Liver function, new function (do not administer if eGFR is less than 30ml/min or ALT is more than 5 times the normal upper limit) 5) paxlovide (antiviral drug)INDICATION (EXPANDED APPLICATION FROM January 21) ADMINISTRATION METHOD Immunosuppressants aged 60 to 40 among mild and moderate patients within 5 days of symptoms (HIV infected, B-cell targeted treatment or solid organ transplant within 1 year) ○ 300 mg (150 mg2 tablets) of nilmatrevir + 100 mg of litonavir twice a day (every 12 hours)※ combination of drug administrator, severe kidney failure, including severe kidney failure, including an irregular pulse (e.g.idone), or antideinflammatory drug (a) (a) heart failure)(MBI>25kg/m2 excess weight (including asthma) or more than oral antisocial cancer) or oral antifunctional anti-virus agent comparisonLagebriopaxlovir PF-07321332/ritonavir Action Mechanical mRNA Replication Inhibitor Proteolytic Enzyme Inhibitor Act 830,000 won per person, 640,000 won per person, 50% 89% of death risk reduction within 5 days / 60 to 40 years old or older * 18 years oldLab F/UCBC (WBC/Lymphocyte Count) AST/ALT, LDH/Ferritin, hsCRP, procalcitonin, d-dimer, RTPCR(ct)